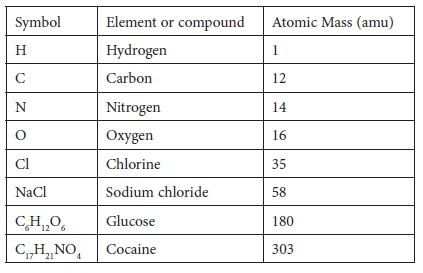
Relative mass on the atomic scale
It is convenient to describe masses on a relative scale of numbers that has no units of ‘u’. Relative mass of an entity is equal to the mass of that entity (u) is equal to 1/12 mass of a carbon 12 atom (1u), which in turn is also equal to a number with no units.
Relative mass of an isotope
These are very close to whole numbers and, for most purposes, are usually quoted as those whole numbers.
E.g.
1 2 12 13 35 37
H = 1 H = 2 C = 12 C= 13 Cl= 35 Cl = 37
Relative atomic mass
Naturally occurring elements exist as a mixture of different isotopes. Th e relative atomic mass of the element will be affected by the relative proportions of the different isotopes.
E.g. About 75% of naturally occurring chlorine is 75% of 35Cl and 25% of 37Cl. Thus the relative atomic mass of Cl is ;
0.75 x 35 + 0.25 x 37 = 35.5.
Relative atomic mass (R.A.M.) of an element
Th e relative atomic mass (R.A.M.) of an element is the average mass of the atoms in the naturally-occurring isotopic mixture of a carbon 12 atom (1u). It can be calculated from knowing the natural isotope abundance.

Where;
• m1, m2, m3 are the masses of the individual isotopes (use accurate values in u if they are given, or use the mass numbers of the isotopes)
• P1, P2, P3 are the percentages of these isotopes in the naturally occurring mixture for this element.
R.A.M. values of elements are oft en expressed to the nearest whole number. E.g. H = 1, Li = 7, C = 12 and Na = 23. But sometimes more accurate values are needed, E.g. Cl = 35.45. Use the values provided on a Periodic Table.